Describe the Properties of Carboxylic Acids
The remainder must be. The amino acids in proteins are α-amino acids which means the amino group is attached to the α-carbon of the carboxylic acid.
The trigonal planar carbon in the carbonyl group can attach to two other substituents leading to several subfamilies aldehydes ketones.
. Structures and Names Humans can synthesize only about half of the needed amino acids. Explain with the use of equations how soap is formed. Multiple soap molecules.
For more information about the α-carbon see Chapter 4 Carboxylic Acids Esters Section 42 Carboxylic Acids. In this equation h is the height of the liquid inside the capillary tube relative to the surface of the liquid outside the tube T is the surface tension of the liquid θ is the contact angle between the liquid and the tube r is the radius of the tube ρ is the density of the liquid and g is the acceleration due to gravity 98 ms 2When the tube is made of a material to which the liquid. Biological significance of fatty acids Soap qualifies as a surfactant due to its molecular structure.
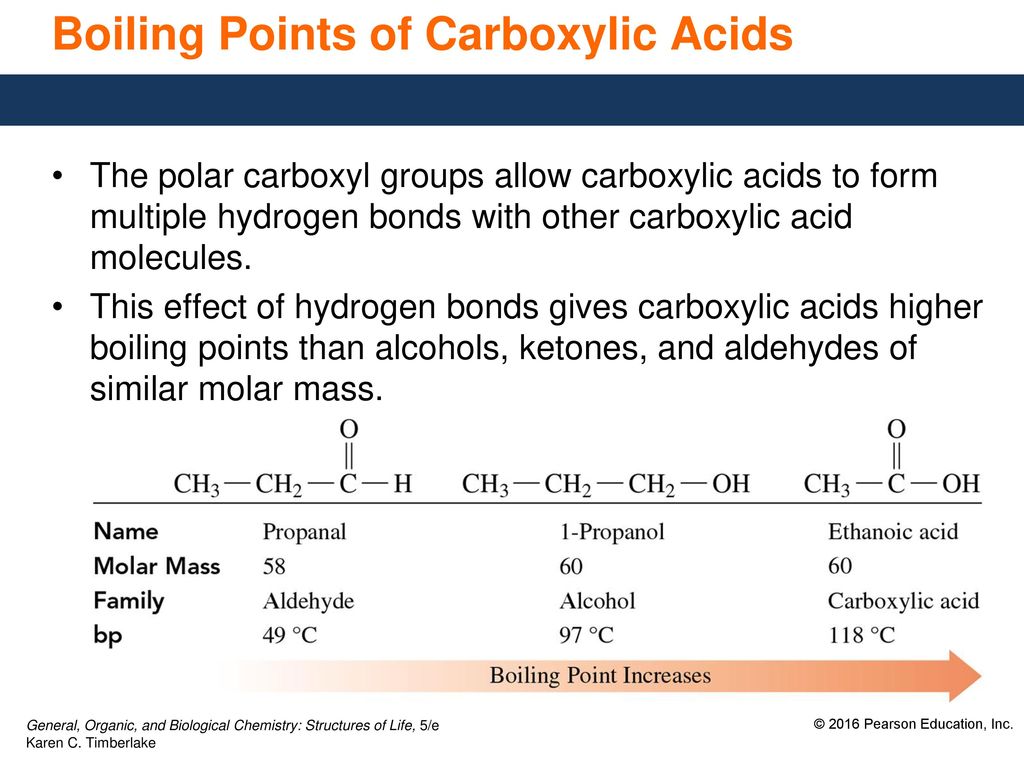
Describe a procedure for making soap. Describe the structure and properties of aldehydes ketones carboxylic acids and esters. The non-polar tail is capable of dissolving non-polar molecules such as oils and grease and the polar head is capable of dissolving polar molecules such as water.
Another class of organic molecules contains a carbon atom connected to an oxygen atom by a double bond commonly called a carbonyl group.

Carboxylic Acids Esters And Other Acid Derivatives Chapter Ppt Download

6 Properties Of Carboxylic Acids Alcohol Carboxylic Acid And Esters

Comments
Post a Comment